Scaleban - Technology/ ZLD Approach
“SCALEBAN” is a unique and non-conventional approach which eliminates the need for conventional technologies available globally to achieve water conservation and ZLD objectives. We are aware that numerous medium and large size cooling towers are used in various industry globally where a lot of fresh water is required for their water makeup requirement on a regular basis.
It is also a fact that cooling towers work on evaporation principle, where we are losing water in the form of water vapors. Therefore, continuous water makeup is required. Globally cooling towers are being operated with lower TDS and conductivity to ensure zero scale, corrosion and biofouling free operation of the entire cooling tower circuit.


Thereby, cooling towers are being operated at very low cycle of concentration (COC) leading to substantial blow down requirement, resulting in additional water requirement for cooling tower makeup (Evaporation + Blow Down). Cooling towers blowdown water is also a part of wastewater treatment plant (WTP) increasing substantial load of WTP along with other wastewater streams coming from various processes. This entire wastewater is being treated in the wastewater treatment plant (ETP) for its safe disposal outside the plant and for achieving Zero Liquid Discharge (ZLD).
Looking at the water scarcity, industries are working on suitable technologies for recycling of wastewater to reduce their fresh water requirement for achieving water conservation and ZLD objectives. However, globally available conventional technologies are very expensive in terms of high CAPEX & OPEX besides high energy requirement leading to high CO2 emission which is a global concern. These available conventional technologies are struggling with wastewater variations affecting system performance and overall objective of industries.
We at Scaleban, understood the ongoing challenges. After extensive research, we innovated a sustainable, green, and cost-effective solution to help industries for achieving their water conservation and ZLD objectives. “SCALEBAN” solution is a combination of “SCALEBAN” equipment, “SCALEBAN” specialty chemicals and “SCALEBAN” Filtration system, by which high TDS wastewater such as ETP treated water, RO reject can be successfully utilize in the cooling towers in place of fresh water.
With our offered solution cooling towers can be successfully operated at very high COC (15-20) with very high TDS up to 300,000 ppm without affecting the plant performance by ensuring zero scale, corrosion and bio-fouling free operations of the heat exchangers including entire cooling tower circuit.
By adopting SCALEBAN Solutions, we achieve following major benefits:
- Successful Utilization of high TDS wastewater in cooling towers
- Achieving water conservation and ZLD objectives on a sustainable basis
- Saving in high CAPEX and OPEX of conventional technologies such as wastewater RO and MEE (evaporator) for achieving ZLD
- Zero freshwater requirement in cooling towers
- High COC operation of cooling towers (15-20) thereby reduction in cooling tower blowdown quantity by 90%
- Ensure zero scale, corrosion and bio fouling free operation of the heat exchangers including entire cooling tower circuit
- Scaleban solution doesn't requires energy to operate thereby reduction in CO₂ emission addressing global concern.
- Contributing in UN SDG goal number 6,7,13 & 14
SCALEBAN WATER CONSERVATION / ZLD APPROACH
How SCALEBAN addresses following concerns while
operating cooling tower with wastewater at High tds/ high coc
Hard Water Scaling
Scaleban equipment is a pipe shaped equipment will be installed just at the inlet of every heat exchanger in the cooling water line which will prevent scaling in the heat exchangers even at very high hardness available in the cooling tower circulation water.
Corrosion
Scaleban speciality chemicals (Corrosion inhibitors) are suitably designed to control corrosion on the different metallurgy in the cooling tower circuit, even at very high TDS, chlorides, sulphates in the cooling tower circulation water.
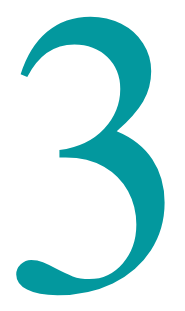
Bio-fouling
Scaleban Specialty chemicals (Biocides) are suitably designed to control biofouling in the cooling tower circuit even with the use of wastewater having COD & BOD.
TSS control
Scaleban Pre-filtration system for cooling tower makeup water & side stream filtration system (SSF) at cooling tower having glass media is used for effective filtration to control suspended solids.
scaleban working principle
Solubility characteristics of SALT IN water
To understand the working process of Scaleban, let us first understand the solubility characteristics of the Calcium & Magnesium salt in water with the rise in temperature. Let us take an example of solubility of sugar in water. Sugar solubility increases with the increase in temperature.
However, in case of Calcium & Magnesium salt the solubility shows a reverse nature with respect to temperature & pH therefore the solubility of Calcium & Magnesium in water decreases with the increase in temperature or pH of water. The relationship can be seen from the graph – 1 & 2
IMPACT OF TEMPERATURE & pH ON SOLUBILITY OF Ca⁺⁺ & Mg⁺⁺
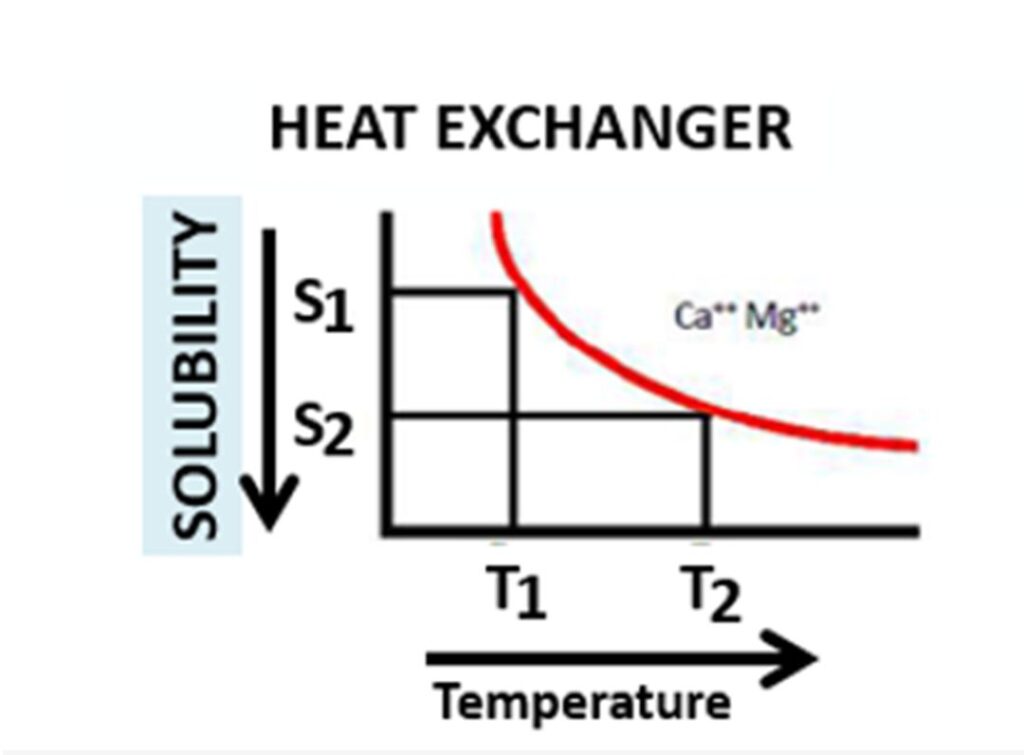
Graph – 1: Relationship between Temperature and Solubility of Ca⁺⁺ & Mg⁺⁺ salt in water
As it is known, the main reason for the formation of scale inside any heat exchangers are due to rise in temperature of cooling water flowing in it. Since at higher temperature; calcium and magnesium salts solubility decreases resulting in formation of scale. A graphical representation of solubility of these salts in water with relation to temperature indicates that with the rise in temperature of water from T1 to T2 the solubility of these salts reduces from S1 to S2 and start precipitation/scaling in the heat exchanger.
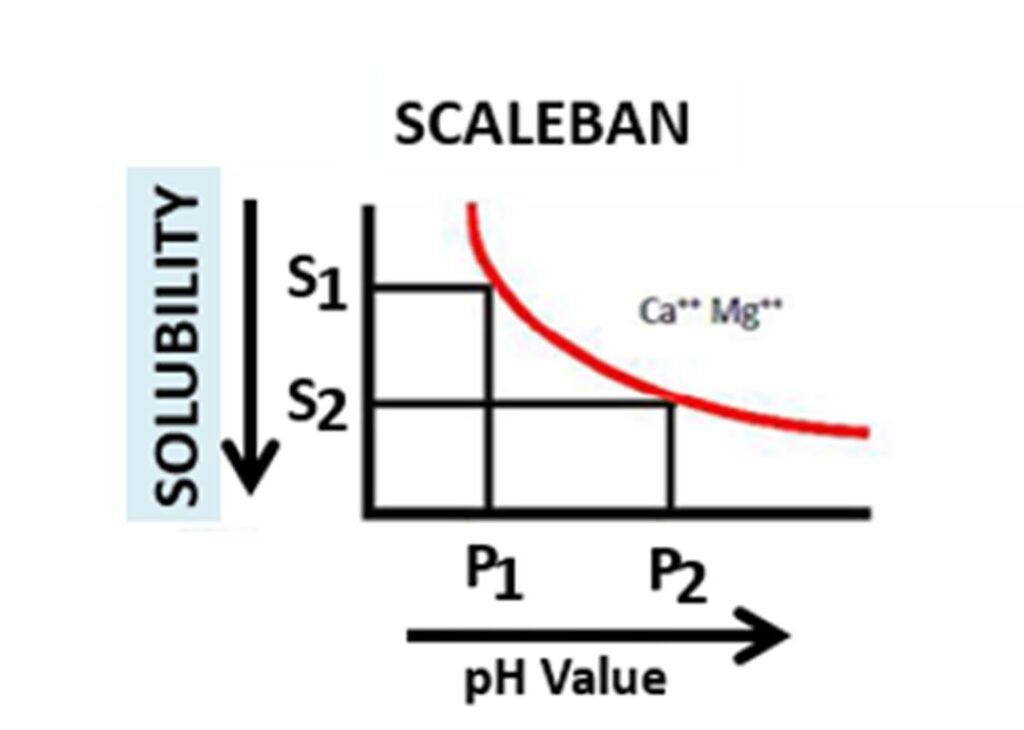
Graph – 2: RELATIONSHIP BETWEEN pH AND SOLUBILITY OF Ca⁺⁺ & Mg⁺⁺ SALT IN WATER
It is also known that the solubility of Calcium & Magnesium salt in water decreases with the increase in pH of water. This can be seen from the second graph. Similar to its tendency to precipitate out dissolved Calcium and Magnesium salt at higher temperature.
SCALEBAN WORKS ON GALVANIC PRINCIPLE

GALVANIC PRINCIPLE
SCALEBAN EQUIPMENT works on Galvanic Principle. SCALEBAN is a pipe shaped mechanical device which houses very specially designed core sintered with number of electronegative elements in their increasing order of electro-negativity in the direction of cooling water flow. SCALEBAN Equipment is installed nearest to the heat exchanger in the cooling water line. Water being a self electrolyte when it passes through SCALEBAN, it acts as an electrolyte.
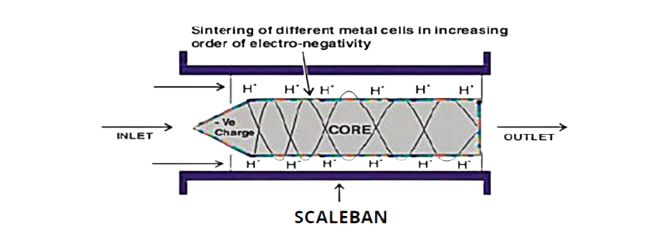
Due to galvanic action, the core gets negatively charged and attracts the lightest ions present in the fluid i.e. Hydrogen ions (H⁺) towards itself. The relationship between pH of water and H⁺ ion is expressed by the formula pH ∝ 1/H⁺. As pH is inversely proportional to H⁺ ions concentration; the concentration of H⁺ ions becoming comparatively less in the water momentarily inside the “SCALEBAN” equipment thereby pH increases and reduces the solubility of Calcium and Magnesium salt in very fine particles having colloidal nature.
These colloidal particles travel along with water, passes through the heat exchangers without forming scales as colloidal particles are non-sticky in nature. Scaling in the heat exchangers only takes place when calcium & magnesium hardness is in dissolve state. SCALEBAN equipment converts the physical nature of hardness causing salts from dissolve to semi-precipitate which does not re-precipitate due to further rise in the temperature in the heat exchanger ensures system scale-free.
inside Scaleban chemical reaction & Technical Working
Scaleban works on Galvanic principle. The role of using Galvanic principle in Scaleban equipment is to create negative charge on the surface of Scaleban metal core which attracts hydrogen ions (H⁺) {lightest ions present in water}. This localized reduction of hydrogen ions concentration in water results in an increase in the pH. As a result, dissolved hardness in the water is transformed into fine colloidal particles. These non-sticky colloidal particles remain suspended in the water and pass through heat exchangers without forming hard scale deposits. This mechanism effectively prevents scaling on heat exchanger surfaces within the cooling water circuit, ensuring improved heat transfer efficiency and system performance.
Mechanism of Action:
Scaleban equipment facilitates the conversion of M alkalinity (bicarbonate ions, HCO₃⁻) to P alkalinity (carbonate ions, CO₃²⁻) by promoting the following reaction, which occurs when the pH rises above 8.3:
HCO₃⁻ + OH⁻ → CO₃²⁻ + H₂O
Impact of increasing pH and Water Chemistry:
1. Conversion of M Alkalinity to P Alkalinity:
- Scaleban equipment promotes the conversion of bicarbonate ions (HCO₃⁻) into carbonate ions (CO₃²⁻) as the pH rises above 8.3. This is achieved through the following reaction: HCO₃⁻ + OH⁻ → CO₃²⁻ + H₂O
- This reaction is critical as it reduces the concentration of hydrogen ions (H⁺), causing the pH to increase and making the water more alkaline.
2. Formation of Colloidal Particles:
- In the alkaline environment created inside the Scaleban Equipment, the carbonate ions (CO₃²⁻) react with calcium (Ca²⁺) and magnesium (Mg²⁺) ions, which are the primary components of hardness. Instead of forming hard, adherent scale, these reactions produce colloidal particles:
■ Calcium Carbonate Formation: Ca²⁺ + CO₃²⁻ → CaCO₃ (colloidal form)
■ Magnesium Hydroxide Formation: Mg²⁺ + 2OH⁻ → Mg(OH)₂ (colloidal form)
Conclusion:
The key chemical equation that drives these changes in pH and alkalinity is:
HCO₃⁻ + OH⁻ → CO₃²⁻ + H₂O
This reaction, facilitated by “SCALEBAN”, is fundamental to preventing the formation of scale while ensuring sustainable water management. Scaleban’s advanced technology exemplifies a cutting- edge approach to industrial water management, prioritizing efficiency and environmental stewardship.
Explore "Scaleban" Case Studies
Corporate Office
#212, Silver Sanchora Castle, R.N.T. Marg, Indore – 452001,
Madhya Pradesh, India
Phone: +91 731 4041974, 4084402
Email: [email protected]
For Enquiries
Phone: +91 9111555403
Email: [email protected]
Email: [email protected]





